Cancers |
您所在的位置:网站首页 › supplementary table s2 › Cancers |
Cancers
|
Next Article in Journal
Integrative Analysis of Next-Generation Sequencing for Next-Generation Cancer Research toward Artificial Intelligence
Next Article in Special Issue
Shorter Leukocyte Telomere Length Is Associated with Worse Survival of Patients with Bladder Cancer and Renal Cell Carcinoma
Previous Article in Journal
Galectin-1 Expression Is Associated with the Response and Survival Following Preoperative Chemoradiotherapy in Locally Advanced Esophageal Squamous Cell Carcinoma
Previous Article in Special Issue
A Prospective Feasibility Trial to Challenge Patient–Derived Pancreatic Cancer Organoids in Predicting Treatment Response
Journals
Active Journals
Find a Journal
Proceedings Series
Topics
Information
For Authors
For Reviewers
For Editors
For Librarians
For Publishers
For Societies
For Conference Organizers
Open Access Policy
Institutional Open Access Program
Special Issues Guidelines
Editorial Process
Research and Publication Ethics
Article Processing Charges
Awards
Testimonials
Author Services
Initiatives
Sciforum
MDPI Books
Preprints
Scilit
SciProfiles
Encyclopedia
JAMS
Proceedings Series
About
Overview
Contact
Careers
News
Blog
Sign In / Sign Up
Notice
clear
Notice
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader. Continue Cancel clearAll articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess. Feature Papers represent the most advanced research with significant potential for high impact in the field. Feature Papers are submitted upon individual invitation or recommendation by the scientific editors and undergo peer review prior to publication. The Feature Paper can be either an original research article, a substantial novel research study that often involves several techniques or approaches, or a comprehensive review paper with concise and precise updates on the latest progress in the field that systematically reviews the most exciting advances in scientific literature. This type of paper provides an outlook on future directions of research or possible applications. Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.  Journals
Active Journals
Find a Journal
Proceedings Series
Topics
Information
For Authors
For Reviewers
For Editors
For Librarians
For Publishers
For Societies
For Conference Organizers
Open Access Policy
Institutional Open Access Program
Special Issues Guidelines
Editorial Process
Research and Publication Ethics
Article Processing Charges
Awards
Testimonials
Author Services
Initiatives
Sciforum
MDPI Books
Preprints
Scilit
SciProfiles
Encyclopedia
JAMS
Proceedings Series
About
Overview
Contact
Careers
News
Blog
Sign In / Sign Up
Submit
Journals
Cancers
Volume 13
Issue 13
10.3390/cancers13133145
Journals
Active Journals
Find a Journal
Proceedings Series
Topics
Information
For Authors
For Reviewers
For Editors
For Librarians
For Publishers
For Societies
For Conference Organizers
Open Access Policy
Institutional Open Access Program
Special Issues Guidelines
Editorial Process
Research and Publication Ethics
Article Processing Charges
Awards
Testimonials
Author Services
Initiatives
Sciforum
MDPI Books
Preprints
Scilit
SciProfiles
Encyclopedia
JAMS
Proceedings Series
About
Overview
Contact
Careers
News
Blog
Sign In / Sign Up
Submit
Journals
Cancers
Volume 13
Issue 13
10.3390/cancers13133145
 Submit to this Journal
Review for this Journal
Edit a Special Issue
►
▼
Article Menu
Article Menu
Academic Editor
Submit to this Journal
Review for this Journal
Edit a Special Issue
►
▼
Article Menu
Article Menu
Academic Editor
 Izumi Horikawa
Subscribe SciFeed
Recommended Articles
Related Info Links
PubMed/Medline
Google Scholar
More by Authors Links
on DOAJ
Walter, K.
Rodriguez-Aznar, E.
Ferreira, M. S. Ventura
Frappart, P.
Dittrich, T.
Tiwary, K.
Meessen, S.
Lerma, L.
Daiss, N.
Schulte, L.
Najafova, Z.
Arnold, F.
Usachov, V.
Azoitei, N.
Erkan, M.
Lechel, A.
Brümmendorf, T. H.
Seufferlein, T.
Kleger, A.
Tabarés, E.
Günes, C.
Johnsen, S. A.
Beier, F.
Sainz, B.
Hermann, P. C.
on Google Scholar
Walter, K.
Rodriguez-Aznar, E.
Ferreira, M. S. Ventura
Frappart, P.
Dittrich, T.
Tiwary, K.
Meessen, S.
Lerma, L.
Daiss, N.
Schulte, L.
Najafova, Z.
Arnold, F.
Usachov, V.
Azoitei, N.
Erkan, M.
Lechel, A.
Brümmendorf, T. H.
Seufferlein, T.
Kleger, A.
Tabarés, E.
Günes, C.
Johnsen, S. A.
Beier, F.
Sainz, B.
Hermann, P. C.
on PubMed
Walter, K.
Rodriguez-Aznar, E.
Ferreira, M. S. Ventura
Frappart, P.
Dittrich, T.
Tiwary, K.
Meessen, S.
Lerma, L.
Daiss, N.
Schulte, L.
Najafova, Z.
Arnold, F.
Usachov, V.
Azoitei, N.
Erkan, M.
Lechel, A.
Brümmendorf, T. H.
Seufferlein, T.
Kleger, A.
Tabarés, E.
Günes, C.
Johnsen, S. A.
Beier, F.
Sainz, B.
Hermann, P. C.
/ajax/scifeed/subscribe
Article Views
Citations
-
Table of Contents
Altmetric
share
Share
announcement
Help
format_quote
Cite
question_answer
Discuss in SciProfiles
thumb_up
...
Endorse
textsms
...
Comment
Need Help?
Support
Izumi Horikawa
Subscribe SciFeed
Recommended Articles
Related Info Links
PubMed/Medline
Google Scholar
More by Authors Links
on DOAJ
Walter, K.
Rodriguez-Aznar, E.
Ferreira, M. S. Ventura
Frappart, P.
Dittrich, T.
Tiwary, K.
Meessen, S.
Lerma, L.
Daiss, N.
Schulte, L.
Najafova, Z.
Arnold, F.
Usachov, V.
Azoitei, N.
Erkan, M.
Lechel, A.
Brümmendorf, T. H.
Seufferlein, T.
Kleger, A.
Tabarés, E.
Günes, C.
Johnsen, S. A.
Beier, F.
Sainz, B.
Hermann, P. C.
on Google Scholar
Walter, K.
Rodriguez-Aznar, E.
Ferreira, M. S. Ventura
Frappart, P.
Dittrich, T.
Tiwary, K.
Meessen, S.
Lerma, L.
Daiss, N.
Schulte, L.
Najafova, Z.
Arnold, F.
Usachov, V.
Azoitei, N.
Erkan, M.
Lechel, A.
Brümmendorf, T. H.
Seufferlein, T.
Kleger, A.
Tabarés, E.
Günes, C.
Johnsen, S. A.
Beier, F.
Sainz, B.
Hermann, P. C.
on PubMed
Walter, K.
Rodriguez-Aznar, E.
Ferreira, M. S. Ventura
Frappart, P.
Dittrich, T.
Tiwary, K.
Meessen, S.
Lerma, L.
Daiss, N.
Schulte, L.
Najafova, Z.
Arnold, F.
Usachov, V.
Azoitei, N.
Erkan, M.
Lechel, A.
Brümmendorf, T. H.
Seufferlein, T.
Kleger, A.
Tabarés, E.
Günes, C.
Johnsen, S. A.
Beier, F.
Sainz, B.
Hermann, P. C.
/ajax/scifeed/subscribe
Article Views
Citations
-
Table of Contents
Altmetric
share
Share
announcement
Help
format_quote
Cite
question_answer
Discuss in SciProfiles
thumb_up
...
Endorse
textsms
...
Comment
Need Help?
Support
Find support for a specific problem in the support section of our website. Get Support FeedbackPlease let us know what you think of our products and services. Give Feedback InformationVisit our dedicated information section to learn more about MDPI. Get Information clear JSmol Viewer clear first_page settings Order Article Reprints Font Type: Arial Georgia Verdana Font Size: Aa Aa Aa Line Spacing: Column Width: Background: Open AccessArticle Telomerase and Pluripotency Factors Jointly Regulate Stemness in Pancreatic Cancer Stem Cells by Karolin Walter 1 Karolin Walter 1 , ,  Eva Rodriguez-Aznar 1, Eva Rodriguez-Aznar 1,  Monica S. Ventura Ferreira 2, Monica S. Ventura Ferreira 2,  Pierre-Olivier Frappart 1,3 Pierre-Olivier Frappart 1,3 , ,  Tabea Dittrich 1, Tabea Dittrich 1,  Kanishka Tiwary 1 Kanishka Tiwary 1 , ,  Sabine Meessen 4, Sabine Meessen 4,  Laura Lerma 5, Laura Lerma 5,  Nora Daiss 1, Nora Daiss 1,  Lucas-Alexander Schulte 1, Lucas-Alexander Schulte 1,  Zeynab Najafova 6, Zeynab Najafova 6,  Frank Arnold 1 Frank Arnold 1 , ,  Valentyn Usachov 1 Valentyn Usachov 1 , ,  Ninel Azoitei 1, Ninel Azoitei 1,  Mert Erkan 7,8, Mert Erkan 7,8,  Andre Lechel 1, Andre Lechel 1,  Tim H. Brümmendorf 2 Tim H. Brümmendorf 2 , ,  Thomas Seufferlein 1, Thomas Seufferlein 1,  Alexander Kleger 1 Alexander Kleger 1 , ,  Enrique Tabarés 5, Enrique Tabarés 5,  Cagatay Günes 4 Cagatay Günes 4 , ,  Steven A. Johnsen 9 Steven A. Johnsen 9 , ,  Fabian Beier 2 Fabian Beier 2 , ,  Bruno Sainz, Jr. 10,11,12 Bruno Sainz, Jr. 10,11,12 and and  Patrick C. Hermann 1,* Patrick C. Hermann 1,* add
Show full author list
remove
Hide full author list
1
Department of Internal Medicine I, University Medical Centre Ulm, 89081 Ulm, Germany
2
Department of Hematology, Oncology, Hemostaseology and Stem Cell Transplantation, University Hospital of the RWTH Aachen, 52062 Aachen, Germany
3
Institute of Toxicology, University Medical Centre of the Johannes Gutenberg University Mainz, 55131 Mainz, Germany
4
Department of Urology, Ulm University, 89081 Ulm, Germany
5
Department of Preventive Medicine, Public Health and Microbiology, Universidad Autónoma de Madrid (UAM), 28049 Madrid, Spain
6
Department of Surgery, University Medical Center Göttingen, 37075 Göttingen, Germany
7
Department of Surgery, Koç University School of Medicine, Istanbul 34450, Turkey
8
Research Center for Translational Medicine, Koç University, Istanbul 34450, Turkey
9
Gene Regulatory Mechanisms and Molecular Epigenetics Lab, Gastroenterology Research, Mayo Clinic, Rochester, MN 55905, USA
10
Department of Biochemistry, Universidad Autónoma de Madrid (UAM), 28049 Madrid, Spain
11
Department of Cancer Biology, Instituto de Investigaciones Biomédicas “Alberto Sols” (IIBM), CSIC-UAM, 28049 Madrid, Spain
12
Chronic Diseases and Cancer, Area 3—Instituto Ramón y Cajal de Investigación Sanitaria (IRYCIS), 28049 Madrid, Spain
add
Show full affiliation list
remove
Hide full affiliation list
*
Author to whom correspondence should be addressed.
Cancers 2021, 13(13), 3145; https://doi.org/10.3390/cancers13133145
Received: 4 May 2021
/
Revised: 14 June 2021
/
Accepted: 18 June 2021
/
Published: 23 June 2021
(This article belongs to the Special Issue The Dual Roles of Telomeres and Telomerase in Aging and Cancer)
Download
Download PDF
Download PDF with Cover
Download XML
Download Epub
Download Supplementary Material
Browse Figures
Versions Notes add
Show full author list
remove
Hide full author list
1
Department of Internal Medicine I, University Medical Centre Ulm, 89081 Ulm, Germany
2
Department of Hematology, Oncology, Hemostaseology and Stem Cell Transplantation, University Hospital of the RWTH Aachen, 52062 Aachen, Germany
3
Institute of Toxicology, University Medical Centre of the Johannes Gutenberg University Mainz, 55131 Mainz, Germany
4
Department of Urology, Ulm University, 89081 Ulm, Germany
5
Department of Preventive Medicine, Public Health and Microbiology, Universidad Autónoma de Madrid (UAM), 28049 Madrid, Spain
6
Department of Surgery, University Medical Center Göttingen, 37075 Göttingen, Germany
7
Department of Surgery, Koç University School of Medicine, Istanbul 34450, Turkey
8
Research Center for Translational Medicine, Koç University, Istanbul 34450, Turkey
9
Gene Regulatory Mechanisms and Molecular Epigenetics Lab, Gastroenterology Research, Mayo Clinic, Rochester, MN 55905, USA
10
Department of Biochemistry, Universidad Autónoma de Madrid (UAM), 28049 Madrid, Spain
11
Department of Cancer Biology, Instituto de Investigaciones Biomédicas “Alberto Sols” (IIBM), CSIC-UAM, 28049 Madrid, Spain
12
Chronic Diseases and Cancer, Area 3—Instituto Ramón y Cajal de Investigación Sanitaria (IRYCIS), 28049 Madrid, Spain
add
Show full affiliation list
remove
Hide full affiliation list
*
Author to whom correspondence should be addressed.
Cancers 2021, 13(13), 3145; https://doi.org/10.3390/cancers13133145
Received: 4 May 2021
/
Revised: 14 June 2021
/
Accepted: 18 June 2021
/
Published: 23 June 2021
(This article belongs to the Special Issue The Dual Roles of Telomeres and Telomerase in Aging and Cancer)
Download
Download PDF
Download PDF with Cover
Download XML
Download Epub
Download Supplementary Material
Browse Figures
Versions Notes
Abstract: Simple SummaryPancreatic ductal adenocarcinoma (PDAC) is an extremely lethal cancer with very limited therapeutic options. Cancer stem cells (CSCs) are essential for propagation of PDAC, but also for its metastatic activity and chemoresistance. To date, it is still unclear how cancer stem cells (CSCs) regulate their ‘stemness’ and self-renewal properties, and to what extent they share common features with normal stem cells. Telomerase regulation is a key factor in stem cell maintenance. Here, we investigate how telomerase regulation affects CSC biology in PDAC, and delineate the mechanisms by which telomerase activity and CSC properties are linked. AbstractTo assess the role of telomerase activity and telomere length in pancreatic CSCs we used different CSC enrichment methods (CD133, ALDH, sphere formation) in primary patient-derived pancreatic cancer cells. We show that CSCs have higher telomerase activity and longer telomeres than bulk tumor cells. Inhibition of telomerase activity, using genetic knockdown or pharmacological inhibitor (BIBR1532), resulted in CSC marker depletion, abrogation of sphere formation in vitro and reduced tumorigenicity in vivo. Furthermore, we identify a positive feedback loop between stemness factors (NANOG, OCT3/4, SOX2, KLF4) and telomerase, which is essential for the self-renewal of CSCs. Disruption of the balance between telomerase activity and stemness factors eliminates CSCs via induction of DNA damage and apoptosis in primary patient-derived pancreatic cancer samples, opening future perspectives to avoid CSC-driven tumor relapse. In the present study, we demonstrate that telomerase regulation is critical for the “stemness” maintenance in pancreatic CSCs and examine the effects of telomerase inhibition as a potential treatment option of pancreatic cancer. This may significantly promote our understanding of PDAC tumor biology and may result in improved treatment for pancreatic cancer patients. Keywords: cancer stem cells; telomerase; telomere length; self-renewal; stemness; pancreatic cancer 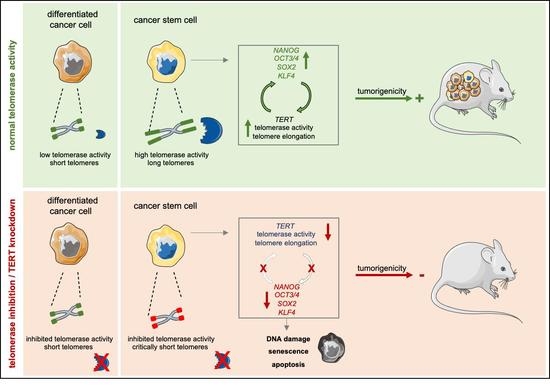
Graphical Abstract 1. IntroductionPancreatic ductal adenocarcinoma (PDAC) is the most frequent and the most lethal form of pancreatic cancer, and it is expected to be the second most frequent cause of cancer-related death by 2030 [1]. Diagnosis of PDAC is frequently delayed due to the absence of early symptoms, pronounced resistance to therapy and early metastatic spread. As a consequence, less than 20% of patients diagnosed with PDAC are eligible for resection [2], the only curative treatment option. Despite growing knowledge about PDAC tumor biology, advances in treatment are still scarce, with FOLFIRINOX and gemcitabine + nab-paclitaxel currently representing the most promising combination chemotherapies [3,4]. The complexity and heterogeneity of pancreatic cancer remains only partially deciphered, and strategies for developing novel and more effective treatments are urgently needed.Cancer stem cells (CSCs) have been implicated in a wide variety of tumors. We and others have previously demonstrated their outstanding importance in pancreatic cancer perpetuation, metastasis, and therapy resistance [5,6]. While self-renewal is a defining characteristic of CSCs [7], the precise mechanism how CSCs maintain their stemness state remains unclear. Therefore, understanding CSCs at the molecular level might reveal targetable principles that could potentially be exploited therapeutically. As telomerase activity and telomere regulation have been proposed to play an essential role in tumor cell maintenance and are considered “hallmarks of cancer” by Hanahan and Weinberg, telomerase activity might present such a potential target.Vertebrate telomeres consist of repetitive TTAGGG DNA sequences. The telomerase complex consists of a catalytic subunit (telomerase reverse transcriptase, TERT) and a telomerase RNA component (TERC). TERC serves as a template for the addition of DNA tandem repeats catalyzed by TERT, which is the rate-limiting factor for telomerase activity [8]. Further factors such as the shelterin proteins TERF1 and TERF2 represent important factors for telomerase regulation [9]. Telomeres protect the ends of the chromosomes from end-to-end fusion and exonucleolytic degradation, preventing genome instability. Importantly, in most human cells telomeric DNA is shortened with each cell division, leading to undetectable telomerase activity in the vast majority of somatic tissues [10] and accumulation of critically short telomeres, subsequently limiting cellular replicative capacity and ultimately resulting in replicative senescence [11].Telomere length stabilization by the reactivation of telomerase or (much less frequently) through alternative mechanisms of telomere lengthening (ALT) results in elevated telomerase activity in 85–90% of human tumors, highlighting this as the prime mechanism to maintain telomere functionality in cancer [12].Several studies have examined the effects of telomerase inhibition on tumor growth. In cancer cell lines, the potent non-nucleosidic telomerase inhibitor BIBR1532 induces telomere shortening, proliferation arrest and senescence [13]. Currently the effects of the clinical grade telomerase inhibitor GRN163L (Imetelstat) are being tested in several tumors, such as breast, lung cancer and myeloma. However, the effects of telomerase inhibition on CSCs have yet to be elucidated.In the present study we investigate the role of telomere regulation and telomerase inhibition in the maintenance of pancreatic CSCs. We demonstrate that CSCs isolated from patient-derived xenografts (PDXs) present higher telomerase activity, resulting in significantly longer telomeres compared to bulk tumor cells. Intriguingly, the lengthening of telomeres is inextricably linked to increased expression of the pluripotency/stemness factors OCT3/4, SOX2, NANOG and KLF4, and is jointly regulated in a previously undescribed positive feedback loop, which is necessary for these cells to escape senescence, and regulates their stemness properties. Furthermore, pharmacological inhibition of telomerase using BIBR1532 as well as genetic knock-down of TERT greatly decreased the CSC frequency of patient-derived PDAC cells in vitro and in vivo by CSC-specific induction of DNA damage and apoptosis. 2. Materials and Methods 2.1. Mice, Transplantation and TreatmentFemale 6–8 week-old athymic Nude-Foxn1nu mice were purchased from Envigo (Gannat, France). For subcutaneous xenografting, single cells were resuspended in 40 µL 1:1 media:Matrigel (Invitrogen, Schwerte, Germany). CSC frequencies and statistical significance of the comparison were determined from extreme limiting dilution assay results using ELDA software [14]. For in vivo treatment, animals received biweekly intraperitoneal (i.p.) injections of doxycycline (1 mg/mL). Tumor size was calculated using the formula (length × width2)/2. All animal experiments were conducted under the ethical and animal protection regulations of the German and Spanish Animal Protection Law and were previously approved by the governmental review board of the state of Baden-Württemberg (V-1347). This included the housing of the animals in specific pathogen-free conditions. 2.2. Primary Pancreatic Cancer Cells and Other Cell LinesPrimary human pancreatic cancer cell lines were generated from established PDX obtained under MTAs (Reference no. I409181220BSMH and I405271505PHMH) from the CNIO, Madrid, Spain, and maintained in culture as described previously [15]. U-2OS and HPDE cells have been previously described [16]. Hek293T cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 1% penicillin/streptomycin and 1% glutamine, in standard conditions (37 °C, 5% CO2). BIBR1532 (Selleckchem, Houston, TX, USA) was used at 80 µM (3 or 7 days treatment, changing media and BIBR1532 every other day), Gemcitabine (Merck, Darmstadt, Germany) was used at 5 µM and Olaparib (Selleckchem) was used at 25 µM (for 72 h) for 3D cell culture unless stated otherwise. 2.3. CSC Enrichment 2.3.1. CD133 and ALDH Fluorescence Activated Cell Sorting (FACS)For CSC enrichment primary pancreatic cancer cells were stained with CD133 (See Supplementary Table S1) or the ALDEFLUORTM Kit (STEMCELLTM Technologies, Vancouver, BC, Canada) was performed. Cell sorting was performed using a FACSAria III (BD Bioscience, Franklin Lakes, NJ, USA). 2.3.2. Sphere CultureSpheres were cultured as described previously [5]. Briefly, cells were cultured in DMEM-F12 supplemented with B-27 (ThermoFisher Scientific, Waltham, MA, USA) and bFGF (Novoprotein, Summit, NJ, USA). 10,000 cells per milliliter were seeded in ultra-low attachment plates (Corning, New York, NY, USA). Spheres were defined as 3-dimensional multicellular structures of ≥40 μm. After 7 days, sphere formation was quantified either manually using a Leica stereomicroscope or using a CASY TT (OLS OMNI Life Science, Bremen, Germany) with a 150 μm capillary. Dead cells and debris were excluded from the quantification. 2.4. Pseudorabies Virus InfectionsThe virulent pseudorabies virus (PRV) strain PRV-NIA3 has been previously described. The parental PRV virus vBecker2 was generated by transfection of pBecker2 plasmid into Hela Tet-Off cells. PRV-TER, a recombinant PRV virus in which the endogenous viral IE180 promoter was substituted with the TERT human tumor promoter, has been detailed previously [16]. For infection of HPDE and Panc185 cells in adherence, 5 × 105 cells were seeded in 6 multi-well plates. Twenty-four hours post seeding, cells were infected with PRV-NIA3, vBecker2 or PRV-TER at a multiplicity of infection (MOI) of 0.1 TCID50/cell. After an absorption period of 2 h at 37 °C, the cells were washed with PBS to remove unabsorbed virus and then incubated for 72 h at 37 °C. Virus yield was determined from total lysates of infected cells on U2OS cells as previously described [16]. For infection of spheres, 7-day-old Panc185 1st generation spheres were trypsinized, cells were counted, and cell suspensions of 5 × 105 cells were seeded in 6 multi-well plates and infected with PRV-NIA3, vBecker2 or PRV-TER at a multiplicity of infection (MOI) of 0.1 TCID50/cell. After an absorption period of 2 h at 37 °C under agitation, cultures were washed with PBS to remove unabsorbed virus, and incubated for 72 h at 37 °C. Virus yield was determined as detailed above. 2.5. Telomerase ActivityFor telomerase activity measurement, the TeloTAGGG telomerase PCR ElisaPLUS kit (Roche, Mannheim, Germany), the telomeric repeat amplification protocol (TRAP) or qRT-PCR based TRAP were performed. Primer sequences are provided in the Supplementary Information (See Supplementary Table S2). 2.6. Organoid CultureTumor pieces of patient-derived xenografts (PDXs) were digested with Accutase® solution (Merck) for 30 min at 37 °C. The cells were then filtered in EASY strainer 100 µM (greiner bio-one, Frickenhausen, Germany) and cultured in Matrigel coated plates containing organoid culture medium as described in Frappart et al. 2020 with 5% growth factor reduced Matrigel (Corning). Media of the organoid culture plates was refreshed every 3–4 days. Organoids were treated with BIBR1532 and gemcitabine at 12.5, 25.0 and 50 µM for 72 h. To determine the number of metabolically active and viable organoids, the CellTiter-Glo® 3D Cell Viability Assay (Promega, Madison, WI, USA) was performed following the manufacturer’s instructions. 2.7. Plasmids, Infection and TransfectionFor RNAi-mediated gene silencing, the pGIPZ and pTRIPZ lentiviral vectors developed by Dr. Greg Hannon (Cold Spring Harbor Laboratory, New York, NY, USA) and Dr. Steve Elledge (Harvard Medical School, Boston, MA, USA) and the corresponding shRNA constructs (GIPZ TERT shRNAs, RHS4531-EG7015 and TRIPZ TERT shRNAs, RHS4740-EG7015) were purchased from Dharmacon (Lafayette, CO, USA). The NANOG reporter lentiviral vector backbone and the sequence of the construct have been previously described [17]. NANOG, OCT3/4, SOX2 and KLF4 were cloned into the PB-EF1-MCS-IRES-RFP plasmid (SBI, Palo Alto, CA, USA). Transposition was performed using Super PiggyBac transposase (SBI), transfection was performed with standard Lipofectamine® 2000 (Invitrogen). 2.8. Flow CytometryAntibodies used in this study are listed in the Supplementary Table S1. For ALDH detection the ALDEFLUORTM kit (STEMCELLTM Technologies) was used. Apoptosis was measured using Annexin V-APC (BD Bioscience) and CellEventTM Caspase-3/7 green detection reagent (Invitrogen). For DNA content staining cells were stained with DAPI. Cellular senescence was measured using the SA-ß-gal kit (BioCat, Heidelberg, Germany). Samples were analyzed by flow cytometry using a LSR II (BD Bioscience), and data were analyzed with FlowJo V10 (Ashland, OR, USA). FACSorting was performed using a FACSAria III (BD Bioscience). 2.9. Q-FISHTelomere Q-FISH was performed as previously described [18]. Fluorescence intensity of the telomeres was quantified with Definiens software (Definiens, Munich, Germany). 2.10. ImmunofluorescencePrimary pancreatic cancer cells were FACSorted, seeded on cover-slips in 6-well dishes, incubated at 37 °C for 12 h, washed with PBS and fixed with 2% PFA for 20 min at room temperature. Coverslips were washed 3 times with PBS and incubated at room temperature for 15 min in 0.7% triton in PBS. 1 h blocking with 5% milk powder in 0.1% TBS-T at room temperature was followed by incubation with the respective antibodies (1:500) in a humidified chamber at 4 °C overnight. Coverslips were then washed 3× with 0.1% TBS-T. Secondary antibodies (1:1000) were incubated at room temperature in a humidified chamber for 2 h, washed and mounted with ProLongTMGold Antifade mounting reagent with DAPI (ThermoFisher Scientific). Images were captured using a Leica TCS SP8-HCS confocal microscope. Antibodies are listed in the Supplementary Table S1. 2.11. RNA Isolation and qRT-PCRTotal RNA was prepared using RNeasy kits with on-column genomic DNA digestion following the manufacturer’s instructions (Qiagen, Hilden, Germany). First strand cDNA was prepared using QuantiTect Reverse Transcription kit (Qiagen). Reactions were performed with QuantiFastSybr Green PCR Kit (Qiagen) using a QuantStudio 3 machine (Applied Biosystems, Waltham, MA, USA). Results were analyzed using the 2-ddCt method and calculated as relative to HPRT expression. Reactions were carried out from at least three independent experiments. Primer sequences are provided in the Supplementary Table S2. 2.12. MTT Assay1000 cells were seeded in a 96-well plate and incubated for 24 h at 37 °C. Cells were incubated for 72 h with the indicated concentrations of the respective drugs and further incubated for 3 h with 5 mg/mL MTT (Merck). Finally, DMSO (Roth, Karlsruhe, Germany) was added, and the optical density was measured at 560 nm using an Infinite Pro 200 plate reader (Tecan, Männedorf, Switzerland). 2.13. Statistical AnalysisResults for continuous variables are presented as means ± SEM. Unless stated otherwise, treatment groups were compared with the one-sided Mann–Whitney-U test, tumor growth dynamics were compared by calculating areas under the curve. Chi-squared tests were used to compare CSC frequencies. Contingency tables were compared using Fisher’s exact test. p-Values 150 measurements per group. In (C–J), data are presented as mean ± SEM. * p ≤ 0.05 (Mann–Whitney-U test). Figure 1. Telomerase activity stabilizes telomere length in pancreatic CSCs: (A) telomere length in primary PDAC cells (red dot) with respect to patient age measured by FlowFISH; (B) schematic illustration showing the CSC enrichment methods for CD133 and ALDEFLUOR via FACS or in sphere culture; (C–E) RT-qPCR analysis of TERT and TERF1 mRNA levels in primary pancreatic cancer stem cells enriched and selected by FACS for CD133 (C), or ALDEFLUOR (D), and cultured as spheres (E) (CSCs) vs. corresponding control (non-CSCs) (n = at least 3 independent experiments). (F) Immunofluorescence staining and quantification for TERT (red) in CD133− and CD133+ FACSorted cells (n = 5 independent experiments). Cells were counterstained with DAPI (nuclear marker, blue), 4× magnification is shown. (G–I) Telomerase activity measurement in CD133 (G) or ALDEFLUOR (H) negative vs. positive cells and in adherent vs. sphere cell-cultures (I) (n = 3 independent FACSortings and sphere culture experiments). (J) Representative pictures of Q-FISH with telomeres (green) and DAPI (blue) and violin plot showing telomere length analysis in CD133 negative (non-CSCs) and positive cells (CSCs) 40× magnification is shown. The mean is depicted in numbers and as black line. n ≥ 3 from independent FACSortings with each >150 measurements per group. In (C–J), data are presented as mean ± SEM. * p ≤ 0.05 (Mann–Whitney-U test).
 Figure 2.
Interplay between pluripotency/stemness factors and telomerase activity in pancreatic CSCs: (A,B) RT-qPCR analysis of pluripotency/stemness-associated genes in primary pancreatic CSCs enriched by CD133 expression (A) or ALDEFLUOR activity (B) vs. CD133− and ALDEFLUOR- non-CSCs (n = at least 3 independent FACSortings). (C–F) Quantitative RT-PCR (C), telomerase activity (D), flow cytometry analysis for CD133 (E) and sphere formation capability (F), compared in NANOG negative cells (white) vs. NANOG positive cells (yellow) using a NANOG-YNL reporter. (G) Schematic illustration and quantification on the production of virions in parental pseudorabies viruses (PRV-NIA3 and vBecker) compared to telomerase activity-dependent virus production (PRV-TER) in adherent HPDE and Panc185 cells as compared to Panc185 spheres. (H) Clonogenic Assay in NANOG-YNL positive and negative cells after withdrawing BIBR1532 (n = ≥3 independent experiments. ns = not statistically significant) (I) Gene expression levels of stemness/pluripotency genes and TERT in HEK293T cells after inducing expression of SOX2, OCT3/4, KLF4 and NANOG, compared to the empty vector control (n = 4 independent experiments). In (A–I), data are represented as mean ± SEM. * p ≤ 0.05 (Mann–Whitney-U test). (J) Telomerase activity after inducing expression of SOX2, OCT3/4, KLF4 and NANOG, compared to the empty vector control. Representative TRAP assays and quantification are depicted.
Figure 2.
Interplay between pluripotency/stemness factors and telomerase activity in pancreatic CSCs: (A,B) RT-qPCR analysis of pluripotency/stemness-associated genes in primary pancreatic CSCs enriched by CD133 expression (A) or ALDEFLUOR activity (B) vs. CD133− and ALDEFLUOR- non-CSCs (n = at least 3 independent FACSortings). (C–F) Quantitative RT-PCR (C), telomerase activity (D), flow cytometry analysis for CD133 (E) and sphere formation capability (F), compared in NANOG negative cells (white) vs. NANOG positive cells (yellow) using a NANOG-YNL reporter. (G) Schematic illustration and quantification on the production of virions in parental pseudorabies viruses (PRV-NIA3 and vBecker) compared to telomerase activity-dependent virus production (PRV-TER) in adherent HPDE and Panc185 cells as compared to Panc185 spheres. (H) Clonogenic Assay in NANOG-YNL positive and negative cells after withdrawing BIBR1532 (n = ≥3 independent experiments. ns = not statistically significant) (I) Gene expression levels of stemness/pluripotency genes and TERT in HEK293T cells after inducing expression of SOX2, OCT3/4, KLF4 and NANOG, compared to the empty vector control (n = 4 independent experiments). In (A–I), data are represented as mean ± SEM. * p ≤ 0.05 (Mann–Whitney-U test). (J) Telomerase activity after inducing expression of SOX2, OCT3/4, KLF4 and NANOG, compared to the empty vector control. Representative TRAP assays and quantification are depicted.
Figure 2.
Interplay between pluripotency/stemness factors and telomerase activity in pancreatic CSCs: (A,B) RT-qPCR analysis of pluripotency/stemness-associated genes in primary pancreatic CSCs enriched by CD133 expression (A) or ALDEFLUOR activity (B) vs. CD133− and ALDEFLUOR- non-CSCs (n = at least 3 independent FACSortings). (C–F) Quantitative RT-PCR (C), telomerase activity (D), flow cytometry analysis for CD133 (E) and sphere formation capability (F), compared in NANOG negative cells (white) vs. NANOG positive cells (yellow) using a NANOG-YNL reporter. (G) Schematic illustration and quantification on the production of virions in parental pseudorabies viruses (PRV-NIA3 and vBecker) compared to telomerase activity-dependent virus production (PRV-TER) in adherent HPDE and Panc185 cells as compared to Panc185 spheres. (H) Clonogenic Assay in NANOG-YNL positive and negative cells after withdrawing BIBR1532 (n = ≥3 independent experiments. ns = not statistically significant) (I) Gene expression levels of stemness/pluripotency genes and TERT in HEK293T cells after inducing expression of SOX2, OCT3/4, KLF4 and NANOG, compared to the empty vector control (n = 4 independent experiments). In (A–I), data are represented as mean ± SEM. * p ≤ 0.05 (Mann–Whitney-U test). (J) Telomerase activity after inducing expression of SOX2, OCT3/4, KLF4 and NANOG, compared to the empty vector control. Representative TRAP assays and quantification are depicted.
Figure 2.
Interplay between pluripotency/stemness factors and telomerase activity in pancreatic CSCs: (A,B) RT-qPCR analysis of pluripotency/stemness-associated genes in primary pancreatic CSCs enriched by CD133 expression (A) or ALDEFLUOR activity (B) vs. CD133− and ALDEFLUOR- non-CSCs (n = at least 3 independent FACSortings). (C–F) Quantitative RT-PCR (C), telomerase activity (D), flow cytometry analysis for CD133 (E) and sphere formation capability (F), compared in NANOG negative cells (white) vs. NANOG positive cells (yellow) using a NANOG-YNL reporter. (G) Schematic illustration and quantification on the production of virions in parental pseudorabies viruses (PRV-NIA3 and vBecker) compared to telomerase activity-dependent virus production (PRV-TER) in adherent HPDE and Panc185 cells as compared to Panc185 spheres. (H) Clonogenic Assay in NANOG-YNL positive and negative cells after withdrawing BIBR1532 (n = ≥3 independent experiments. ns = not statistically significant) (I) Gene expression levels of stemness/pluripotency genes and TERT in HEK293T cells after inducing expression of SOX2, OCT3/4, KLF4 and NANOG, compared to the empty vector control (n = 4 independent experiments). In (A–I), data are represented as mean ± SEM. * p ≤ 0.05 (Mann–Whitney-U test). (J) Telomerase activity after inducing expression of SOX2, OCT3/4, KLF4 and NANOG, compared to the empty vector control. Representative TRAP assays and quantification are depicted.

 Figure 3.
Targeting telomerase activity with a small molecule inhibitor (BIBR1532): (A,B) The effects of BIBR1532 treatment on telomerase activity (A), and telomere length (B) in CD133− cells (blue) and in the CD133+ CSC population (red) were quantified. For illustrative purposes, the telomere length measurements of Figure 1H were re-used here (indicated by transparent color). The mean is depicted in numbers and as black line, >150 measurements per group. (C) Quantification of gH2AX foci (>50 cells per group) in CD133− and CD133+ cells after 7 days vehicle or BIBR1532 treatment. Quantification and representative pictures of immunofluorescence staining of gH2AX foci are provided. (D) Senescent cells quantified by flow cytometry staining for SA-beta-Gal in CD133− (non-CSCs) and CD133+ (CSCs) after BIBR1532 or solvent treatment (7 days). (E,F) Apoptosis quantified by flow cytometry using double staining for CD133 and AnnexinV or (E) Caspase3/7 (F). In (A–F), data are represented as mean ± SEM. n = 3 independent experiments. * p ≤ 0.05 (Mann–Whitney-U test), ns = not statistically significant.
Figure 3.
Targeting telomerase activity with a small molecule inhibitor (BIBR1532): (A,B) The effects of BIBR1532 treatment on telomerase activity (A), and telomere length (B) in CD133− cells (blue) and in the CD133+ CSC population (red) were quantified. For illustrative purposes, the telomere length measurements of Figure 1H were re-used here (indicated by transparent color). The mean is depicted in numbers and as black line, >150 measurements per group. (C) Quantification of gH2AX foci (>50 cells per group) in CD133− and CD133+ cells after 7 days vehicle or BIBR1532 treatment. Quantification and representative pictures of immunofluorescence staining of gH2AX foci are provided. (D) Senescent cells quantified by flow cytometry staining for SA-beta-Gal in CD133− (non-CSCs) and CD133+ (CSCs) after BIBR1532 or solvent treatment (7 days). (E,F) Apoptosis quantified by flow cytometry using double staining for CD133 and AnnexinV or (E) Caspase3/7 (F). In (A–F), data are represented as mean ± SEM. n = 3 independent experiments. * p ≤ 0.05 (Mann–Whitney-U test), ns = not statistically significant.
Figure 3.
Targeting telomerase activity with a small molecule inhibitor (BIBR1532): (A,B) The effects of BIBR1532 treatment on telomerase activity (A), and telomere length (B) in CD133− cells (blue) and in the CD133+ CSC population (red) were quantified. For illustrative purposes, the telomere length measurements of Figure 1H were re-used here (indicated by transparent color). The mean is depicted in numbers and as black line, >150 measurements per group. (C) Quantification of gH2AX foci (>50 cells per group) in CD133− and CD133+ cells after 7 days vehicle or BIBR1532 treatment. Quantification and representative pictures of immunofluorescence staining of gH2AX foci are provided. (D) Senescent cells quantified by flow cytometry staining for SA-beta-Gal in CD133− (non-CSCs) and CD133+ (CSCs) after BIBR1532 or solvent treatment (7 days). (E,F) Apoptosis quantified by flow cytometry using double staining for CD133 and AnnexinV or (E) Caspase3/7 (F). In (A–F), data are represented as mean ± SEM. n = 3 independent experiments. * p ≤ 0.05 (Mann–Whitney-U test), ns = not statistically significant.
Figure 3.
Targeting telomerase activity with a small molecule inhibitor (BIBR1532): (A,B) The effects of BIBR1532 treatment on telomerase activity (A), and telomere length (B) in CD133− cells (blue) and in the CD133+ CSC population (red) were quantified. For illustrative purposes, the telomere length measurements of Figure 1H were re-used here (indicated by transparent color). The mean is depicted in numbers and as black line, >150 measurements per group. (C) Quantification of gH2AX foci (>50 cells per group) in CD133− and CD133+ cells after 7 days vehicle or BIBR1532 treatment. Quantification and representative pictures of immunofluorescence staining of gH2AX foci are provided. (D) Senescent cells quantified by flow cytometry staining for SA-beta-Gal in CD133− (non-CSCs) and CD133+ (CSCs) after BIBR1532 or solvent treatment (7 days). (E,F) Apoptosis quantified by flow cytometry using double staining for CD133 and AnnexinV or (E) Caspase3/7 (F). In (A–F), data are represented as mean ± SEM. n = 3 independent experiments. * p ≤ 0.05 (Mann–Whitney-U test), ns = not statistically significant.

 Figure 4.
Telomerase inhibition as treatment strategy for pancreatic cancer (stem) cells: (A) Effects of the small molecule telomerase inhibitor BIBR1532 on the expression of TERT and pluripotency-associated genes as measured by RT-qPCR (n = 4 independent experiments). (B) Flow cytometry analyses for CD133 or ALDEFLUOR after BIBR1532 or solvent treatment (n = 3 independent experiments). (C) Quantification and representative pictures of spheres after BIBR1532 treatment (n = 3 independent experiments). (D) Schematic overview of BIBR1532 pre-treatment and in vivo experiment, number of tumorigenic cells within the whole population shown as cancer stem cell (CSC) frequencies as determined by extreme limiting dilution assays (ELDA) in nude mice, and tumor volume measured after injection of 100,000 BIBR1532 or solvent treated Panc215 and Panc354 cells (n ≥ 4 mice for each group). (E) Viability of PDX-derived organoids treated with gemcitabine and BIBR1532 at the indicated concentrations. (F) Quantification and representative pictures of sphere formation assays after single agent or combination treatment with gemcitabine, Olaparib, and BIBR1532 (n = 5 independent experiments). In (A–C,E,F) data are represented as mean ± SEM. * p ≤ 0.05 (Mann–Whitney-U test). In (D) data are represented as mean ± SEM. * p ≤ 0.05 (Area under the curve).
Figure 4.
Telomerase inhibition as treatment strategy for pancreatic cancer (stem) cells: (A) Effects of the small molecule telomerase inhibitor BIBR1532 on the expression of TERT and pluripotency-associated genes as measured by RT-qPCR (n = 4 independent experiments). (B) Flow cytometry analyses for CD133 or ALDEFLUOR after BIBR1532 or solvent treatment (n = 3 independent experiments). (C) Quantification and representative pictures of spheres after BIBR1532 treatment (n = 3 independent experiments). (D) Schematic overview of BIBR1532 pre-treatment and in vivo experiment, number of tumorigenic cells within the whole population shown as cancer stem cell (CSC) frequencies as determined by extreme limiting dilution assays (ELDA) in nude mice, and tumor volume measured after injection of 100,000 BIBR1532 or solvent treated Panc215 and Panc354 cells (n ≥ 4 mice for each group). (E) Viability of PDX-derived organoids treated with gemcitabine and BIBR1532 at the indicated concentrations. (F) Quantification and representative pictures of sphere formation assays after single agent or combination treatment with gemcitabine, Olaparib, and BIBR1532 (n = 5 independent experiments). In (A–C,E,F) data are represented as mean ± SEM. * p ≤ 0.05 (Mann–Whitney-U test). In (D) data are represented as mean ± SEM. * p ≤ 0.05 (Area under the curve).
Figure 4.
Telomerase inhibition as treatment strategy for pancreatic cancer (stem) cells: (A) Effects of the small molecule telomerase inhibitor BIBR1532 on the expression of TERT and pluripotency-associated genes as measured by RT-qPCR (n = 4 independent experiments). (B) Flow cytometry analyses for CD133 or ALDEFLUOR after BIBR1532 or solvent treatment (n = 3 independent experiments). (C) Quantification and representative pictures of spheres after BIBR1532 treatment (n = 3 independent experiments). (D) Schematic overview of BIBR1532 pre-treatment and in vivo experiment, number of tumorigenic cells within the whole population shown as cancer stem cell (CSC) frequencies as determined by extreme limiting dilution assays (ELDA) in nude mice, and tumor volume measured after injection of 100,000 BIBR1532 or solvent treated Panc215 and Panc354 cells (n ≥ 4 mice for each group). (E) Viability of PDX-derived organoids treated with gemcitabine and BIBR1532 at the indicated concentrations. (F) Quantification and representative pictures of sphere formation assays after single agent or combination treatment with gemcitabine, Olaparib, and BIBR1532 (n = 5 independent experiments). In (A–C,E,F) data are represented as mean ± SEM. * p ≤ 0.05 (Mann–Whitney-U test). In (D) data are represented as mean ± SEM. * p ≤ 0.05 (Area under the curve).
Figure 4.
Telomerase inhibition as treatment strategy for pancreatic cancer (stem) cells: (A) Effects of the small molecule telomerase inhibitor BIBR1532 on the expression of TERT and pluripotency-associated genes as measured by RT-qPCR (n = 4 independent experiments). (B) Flow cytometry analyses for CD133 or ALDEFLUOR after BIBR1532 or solvent treatment (n = 3 independent experiments). (C) Quantification and representative pictures of spheres after BIBR1532 treatment (n = 3 independent experiments). (D) Schematic overview of BIBR1532 pre-treatment and in vivo experiment, number of tumorigenic cells within the whole population shown as cancer stem cell (CSC) frequencies as determined by extreme limiting dilution assays (ELDA) in nude mice, and tumor volume measured after injection of 100,000 BIBR1532 or solvent treated Panc215 and Panc354 cells (n ≥ 4 mice for each group). (E) Viability of PDX-derived organoids treated with gemcitabine and BIBR1532 at the indicated concentrations. (F) Quantification and representative pictures of sphere formation assays after single agent or combination treatment with gemcitabine, Olaparib, and BIBR1532 (n = 5 independent experiments). In (A–C,E,F) data are represented as mean ± SEM. * p ≤ 0.05 (Mann–Whitney-U test). In (D) data are represented as mean ± SEM. * p ≤ 0.05 (Area under the curve).

 Figure 5.
Knockdown of TERT diminishes pancreatic cancer stem cells: (A,B) Gene expression of TERT (A) and telomerase activity (B) in Panc215 and Panc354 cells transduced with shRNA-340159 and shRNA-340160 compared to scrambled (shSCR) control. (C) Expression levels of stemness/pluripotency-associated genes using shRNA-340160 (shTERT) for TERT mediated knockdown in Panc215 and Panc354 cells. (D,E) Flow cytometry analyses for CD133 (D) and sphere formation (E) upon TERT knock-down (KD) compared to scrambled (shSCR) control. (F) Quantification of apoptosis in shSCR and shTERT transduced cells (Panc215 and Panc354) using flow cytometry analysis for AnnexinV (n = at least 3 independent experiments). (G) In vivo tumor-initiating potential with CSC frequencies (number of tumorigenic cells within the whole population) as determined by ELDA in nude mice after TERT-KD compared to scrambled control (n = 8 animals); (H) Schematic illustration of in vivo experiment with cells carrying an inducible TERT-KD (Dox shTERT) or scrambled (Dox SCR) control construct. Cells were treated with doxycycline (DOX) 7 days before s.c. xenografting in nude mice. Graph shows time-dependent growth of subcutaneously engrafted tumors (n = 4 mice per group). (I) Schematic illustration of in vivo experiment and visual representation of time-dependent tumor growth of subcutaneously (s.c.) engrafted tumors arising from TERT-KD and SCR cells, over the course of doxycycline treatment 28 days after s.c. xenografting in nude mice (n = 6 mice per group). Tumor growth is depicted until the first control mice had to be sacrificed. 4× magnification is shown (J) Gene expression of TERT and telomerase activity compared in TERT-KD and SCR tumors (induced with DOX at day 28) after sacrificing mice 43 days after xenografting. (K) representative H&E staining of at day 43 explanted TERT-KD and scrambled tumors (induced with DOX at day 28). In (A–G,J) data are represented as mean ± SEM. * p ≤ 0.05 (Mann–Whitney-U test). In (H,I) data are represented as mean ± SEM. * p ≤ 0.05 (Area under the curve).
Figure 5.
Knockdown of TERT diminishes pancreatic cancer stem cells: (A,B) Gene expression of TERT (A) and telomerase activity (B) in Panc215 and Panc354 cells transduced with shRNA-340159 and shRNA-340160 compared to scrambled (shSCR) control. (C) Expression levels of stemness/pluripotency-associated genes using shRNA-340160 (shTERT) for TERT mediated knockdown in Panc215 and Panc354 cells. (D,E) Flow cytometry analyses for CD133 (D) and sphere formation (E) upon TERT knock-down (KD) compared to scrambled (shSCR) control. (F) Quantification of apoptosis in shSCR and shTERT transduced cells (Panc215 and Panc354) using flow cytometry analysis for AnnexinV (n = at least 3 independent experiments). (G) In vivo tumor-initiating potential with CSC frequencies (number of tumorigenic cells within the whole population) as determined by ELDA in nude mice after TERT-KD compared to scrambled control (n = 8 animals); (H) Schematic illustration of in vivo experiment with cells carrying an inducible TERT-KD (Dox shTERT) or scrambled (Dox SCR) control construct. Cells were treated with doxycycline (DOX) 7 days before s.c. xenografting in nude mice. Graph shows time-dependent growth of subcutaneously engrafted tumors (n = 4 mice per group). (I) Schematic illustration of in vivo experiment and visual representation of time-dependent tumor growth of subcutaneously (s.c.) engrafted tumors arising from TERT-KD and SCR cells, over the course of doxycycline treatment 28 days after s.c. xenografting in nude mice (n = 6 mice per group). Tumor growth is depicted until the first control mice had to be sacrificed. 4× magnification is shown (J) Gene expression of TERT and telomerase activity compared in TERT-KD and SCR tumors (induced with DOX at day 28) after sacrificing mice 43 days after xenografting. (K) representative H&E staining of at day 43 explanted TERT-KD and scrambled tumors (induced with DOX at day 28). In (A–G,J) data are represented as mean ± SEM. * p ≤ 0.05 (Mann–Whitney-U test). In (H,I) data are represented as mean ± SEM. * p ≤ 0.05 (Area under the curve).
Figure 5.
Knockdown of TERT diminishes pancreatic cancer stem cells: (A,B) Gene expression of TERT (A) and telomerase activity (B) in Panc215 and Panc354 cells transduced with shRNA-340159 and shRNA-340160 compared to scrambled (shSCR) control. (C) Expression levels of stemness/pluripotency-associated genes using shRNA-340160 (shTERT) for TERT mediated knockdown in Panc215 and Panc354 cells. (D,E) Flow cytometry analyses for CD133 (D) and sphere formation (E) upon TERT knock-down (KD) compared to scrambled (shSCR) control. (F) Quantification of apoptosis in shSCR and shTERT transduced cells (Panc215 and Panc354) using flow cytometry analysis for AnnexinV (n = at least 3 independent experiments). (G) In vivo tumor-initiating potential with CSC frequencies (number of tumorigenic cells within the whole population) as determined by ELDA in nude mice after TERT-KD compared to scrambled control (n = 8 animals); (H) Schematic illustration of in vivo experiment with cells carrying an inducible TERT-KD (Dox shTERT) or scrambled (Dox SCR) control construct. Cells were treated with doxycycline (DOX) 7 days before s.c. xenografting in nude mice. Graph shows time-dependent growth of subcutaneously engrafted tumors (n = 4 mice per group). (I) Schematic illustration of in vivo experiment and visual representation of time-dependent tumor growth of subcutaneously (s.c.) engrafted tumors arising from TERT-KD and SCR cells, over the course of doxycycline treatment 28 days after s.c. xenografting in nude mice (n = 6 mice per group). Tumor growth is depicted until the first control mice had to be sacrificed. 4× magnification is shown (J) Gene expression of TERT and telomerase activity compared in TERT-KD and SCR tumors (induced with DOX at day 28) after sacrificing mice 43 days after xenografting. (K) representative H&E staining of at day 43 explanted TERT-KD and scrambled tumors (induced with DOX at day 28). In (A–G,J) data are represented as mean ± SEM. * p ≤ 0.05 (Mann–Whitney-U test). In (H,I) data are represented as mean ± SEM. * p ≤ 0.05 (Area under the curve).
Figure 5.
Knockdown of TERT diminishes pancreatic cancer stem cells: (A,B) Gene expression of TERT (A) and telomerase activity (B) in Panc215 and Panc354 cells transduced with shRNA-340159 and shRNA-340160 compared to scrambled (shSCR) control. (C) Expression levels of stemness/pluripotency-associated genes using shRNA-340160 (shTERT) for TERT mediated knockdown in Panc215 and Panc354 cells. (D,E) Flow cytometry analyses for CD133 (D) and sphere formation (E) upon TERT knock-down (KD) compared to scrambled (shSCR) control. (F) Quantification of apoptosis in shSCR and shTERT transduced cells (Panc215 and Panc354) using flow cytometry analysis for AnnexinV (n = at least 3 independent experiments). (G) In vivo tumor-initiating potential with CSC frequencies (number of tumorigenic cells within the whole population) as determined by ELDA in nude mice after TERT-KD compared to scrambled control (n = 8 animals); (H) Schematic illustration of in vivo experiment with cells carrying an inducible TERT-KD (Dox shTERT) or scrambled (Dox SCR) control construct. Cells were treated with doxycycline (DOX) 7 days before s.c. xenografting in nude mice. Graph shows time-dependent growth of subcutaneously engrafted tumors (n = 4 mice per group). (I) Schematic illustration of in vivo experiment and visual representation of time-dependent tumor growth of subcutaneously (s.c.) engrafted tumors arising from TERT-KD and SCR cells, over the course of doxycycline treatment 28 days after s.c. xenografting in nude mice (n = 6 mice per group). Tumor growth is depicted until the first control mice had to be sacrificed. 4× magnification is shown (J) Gene expression of TERT and telomerase activity compared in TERT-KD and SCR tumors (induced with DOX at day 28) after sacrificing mice 43 days after xenografting. (K) representative H&E staining of at day 43 explanted TERT-KD and scrambled tumors (induced with DOX at day 28). In (A–G,J) data are represented as mean ± SEM. * p ≤ 0.05 (Mann–Whitney-U test). In (H,I) data are represented as mean ± SEM. * p ≤ 0.05 (Area under the curve).
 Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
MDPI and ACS Style
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
MDPI and ACS Style
Walter, K.; Rodriguez-Aznar, E.; Ferreira, M.S.V.; Frappart, P.-O.; Dittrich, T.; Tiwary, K.; Meessen, S.; Lerma, L.; Daiss, N.; Schulte, L.-A.; Najafova, Z.; Arnold, F.; Usachov, V.; Azoitei, N.; Erkan, M.; Lechel, A.; Brümmendorf, T.H.; Seufferlein, T.; Kleger, A.; Tabarés, E.; Günes, C.; Johnsen, S.A.; Beier, F.; Sainz, B., Jr.; Hermann, P.C. Telomerase and Pluripotency Factors Jointly Regulate Stemness in Pancreatic Cancer Stem Cells. Cancers 2021, 13, 3145. https://doi.org/10.3390/cancers13133145 AMA StyleWalter K, Rodriguez-Aznar E, Ferreira MSV, Frappart P-O, Dittrich T, Tiwary K, Meessen S, Lerma L, Daiss N, Schulte L-A, Najafova Z, Arnold F, Usachov V, Azoitei N, Erkan M, Lechel A, Brümmendorf TH, Seufferlein T, Kleger A, Tabarés E, Günes C, Johnsen SA, Beier F, Sainz B Jr., Hermann PC. Telomerase and Pluripotency Factors Jointly Regulate Stemness in Pancreatic Cancer Stem Cells. Cancers. 2021; 13(13):3145. https://doi.org/10.3390/cancers13133145 Chicago/Turabian StyleWalter, Karolin, Eva Rodriguez-Aznar, Monica S. Ventura Ferreira, Pierre-Olivier Frappart, Tabea Dittrich, Kanishka Tiwary, Sabine Meessen, Laura Lerma, Nora Daiss, Lucas-Alexander Schulte, Zeynab Najafova, Frank Arnold, Valentyn Usachov, Ninel Azoitei, Mert Erkan, Andre Lechel, Tim H. Brümmendorf, Thomas Seufferlein, Alexander Kleger, Enrique Tabarés, Cagatay Günes, Steven A. Johnsen, Fabian Beier, Bruno Sainz Jr., and Patrick C. Hermann. 2021. "Telomerase and Pluripotency Factors Jointly Regulate Stemness in Pancreatic Cancer Stem Cells" Cancers 13, no. 13: 3145. https://doi.org/10.3390/cancers13133145 Find Other Styles Note that from the first issue of 2016, this journal uses article numbers instead of page numbers. See further details here. Article Metrics No No Article Access Statistics For more information on the journal statistics, click here. Multiple requests from the same IP address are counted as one view. Supplementary Material Supplementary File 1:ZIP-Document (ZIP, 362 KiB) clear Zoom | Orient | As Lines | As Sticks | As Cartoon | As Surface | Previous Scene | Next Scene Cite Export citation file: BibTeX | EndNote | RIS MDPI and ACS StyleWalter, K.; Rodriguez-Aznar, E.; Ferreira, M.S.V.; Frappart, P.-O.; Dittrich, T.; Tiwary, K.; Meessen, S.; Lerma, L.; Daiss, N.; Schulte, L.-A.; Najafova, Z.; Arnold, F.; Usachov, V.; Azoitei, N.; Erkan, M.; Lechel, A.; Brümmendorf, T.H.; Seufferlein, T.; Kleger, A.; Tabarés, E.; Günes, C.; Johnsen, S.A.; Beier, F.; Sainz, B., Jr.; Hermann, P.C. Telomerase and Pluripotency Factors Jointly Regulate Stemness in Pancreatic Cancer Stem Cells. Cancers 2021, 13, 3145. https://doi.org/10.3390/cancers13133145 AMA StyleWalter K, Rodriguez-Aznar E, Ferreira MSV, Frappart P-O, Dittrich T, Tiwary K, Meessen S, Lerma L, Daiss N, Schulte L-A, Najafova Z, Arnold F, Usachov V, Azoitei N, Erkan M, Lechel A, Brümmendorf TH, Seufferlein T, Kleger A, Tabarés E, Günes C, Johnsen SA, Beier F, Sainz B Jr., Hermann PC. Telomerase and Pluripotency Factors Jointly Regulate Stemness in Pancreatic Cancer Stem Cells. Cancers. 2021; 13(13):3145. https://doi.org/10.3390/cancers13133145 Chicago/Turabian StyleWalter, Karolin, Eva Rodriguez-Aznar, Monica S. Ventura Ferreira, Pierre-Olivier Frappart, Tabea Dittrich, Kanishka Tiwary, Sabine Meessen, Laura Lerma, Nora Daiss, Lucas-Alexander Schulte, Zeynab Najafova, Frank Arnold, Valentyn Usachov, Ninel Azoitei, Mert Erkan, Andre Lechel, Tim H. Brümmendorf, Thomas Seufferlein, Alexander Kleger, Enrique Tabarés, Cagatay Günes, Steven A. Johnsen, Fabian Beier, Bruno Sainz Jr., and Patrick C. Hermann. 2021. "Telomerase and Pluripotency Factors Jointly Regulate Stemness in Pancreatic Cancer Stem Cells" Cancers 13, no. 13: 3145. https://doi.org/10.3390/cancers13133145 Find Other Styles Note that from the first issue of 2016, this journal uses article numbers instead of page numbers. See further details here. clear Cancers, EISSN 2072-6694, Published by MDPI RSS Content Alert Further Information Article Processing Charges Pay an Invoice Open Access Policy Contact MDPI Jobs at MDPI Guidelines For Authors For Reviewers For Editors For Librarians For Publishers For Societies For Conference Organizers MDPI Initiatives Sciforum MDPI Books Preprints Scilit SciProfiles Encyclopedia JAMS Proceedings Series Follow MDPI LinkedIn Facebook Twitter © 1996-2023 MDPI (Basel, Switzerland) unless otherwise stated
Disclaimer
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely
those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or
the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas,
methods, instructions or products referred to in the content.
Terms and Conditions
Privacy Policy
We use cookies on our website to ensure you get the best experience.
Read more about our cookies here.
Accept
We have just recently launched a new version of our website.
Help us to further improve by taking part in this short 5 minute survey
here.
here.
Never show this again
Share Link
Copy
clear
Share
https://www.mdpi.com/1161838
clear
Back to TopTop
© 1996-2023 MDPI (Basel, Switzerland) unless otherwise stated
Disclaimer
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely
those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or
the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas,
methods, instructions or products referred to in the content.
Terms and Conditions
Privacy Policy
We use cookies on our website to ensure you get the best experience.
Read more about our cookies here.
Accept
We have just recently launched a new version of our website.
Help us to further improve by taking part in this short 5 minute survey
here.
here.
Never show this again
Share Link
Copy
clear
Share
https://www.mdpi.com/1161838
clear
Back to TopTop
|
【本文地址】